GP-001 to GP-012 Series Empower Safer Surgical Energy Management**
— Goceng Medical announced the official release of its upgraded GP-001 to GP-012 Neutral Electrode(grounding pads) Series, delivering a full-spectrum solution for modern operating rooms.
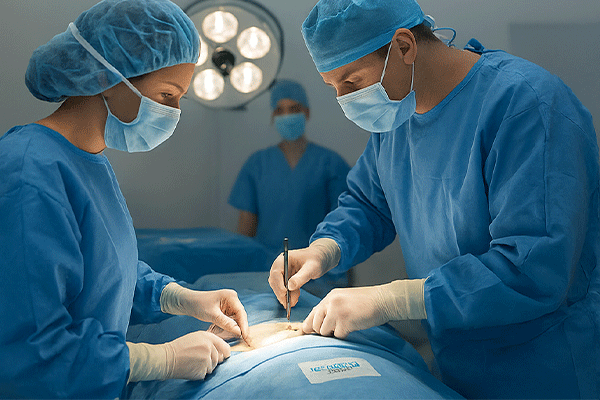
With the growing reliance on electrosurgical units (ESU), diathermy, and high-frequency energy devices, neutral electrodes—also known as patient return pads—play a crucial role in protecting patients from thermal injuries. The new Goceng Medical series introduces enhanced safety, adaptability, and international device compatibility.
Meeting the Evolving Needs of Global Operating Rooms
Different surgical disciplines, patient body types, and OR environments demand increasingly specialized return electrode solutions. The GP-001 to GP-012 series was designed based on real clinical use cases from general surgery, gynecology, urology, orthopedics, pediatric surgery, and emergency medicine.
Key application scenarios include:
1. Long-duration oncologic and general surgeries requiring strong adhesion and stable conductivity
2. Gynecology and obstetrics, with softer materials for sensitive skin
3. Urology and orthopedic surgeries, where moisture resistance and strong bonding are crucial
4. Pediatric surgeries, requiring smaller, gentler hydrogel formulations
5. Emergency operations, where fast application and universal compatibility are essential
6. International markets, with full REM/Split-Type monitoring support for Valleylab, ConMed, Erbe, and other ESU brands
Technology Highlights of the GP-001 ~ GP-012 Series
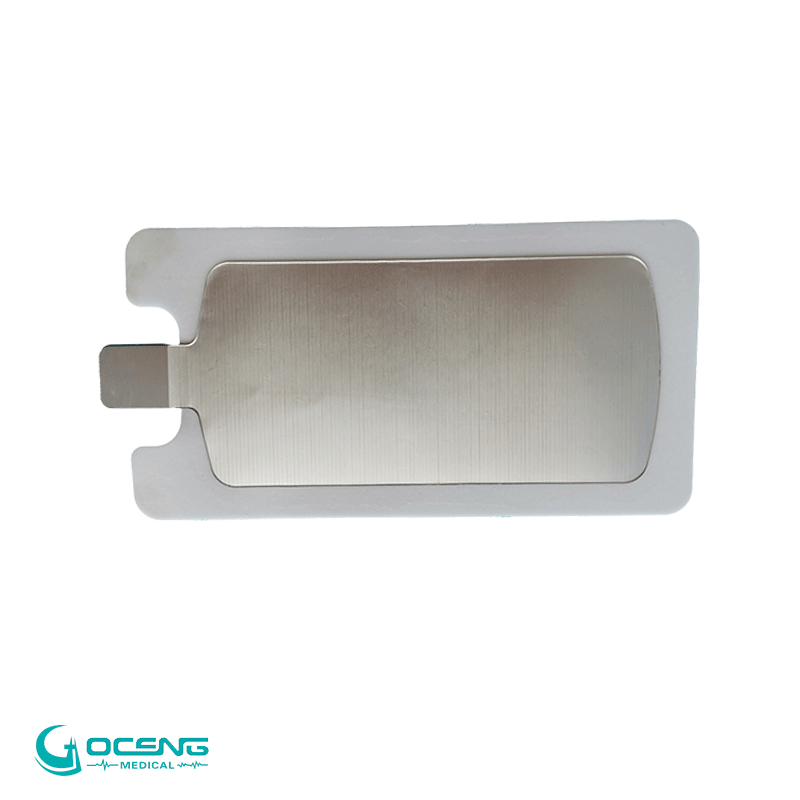
1. Advanced Hydrogel Conductive System
High electrical conductivity
Long-lasting moisture retention
No residue after removal
Adult, pediatric, and sensitive-skin options
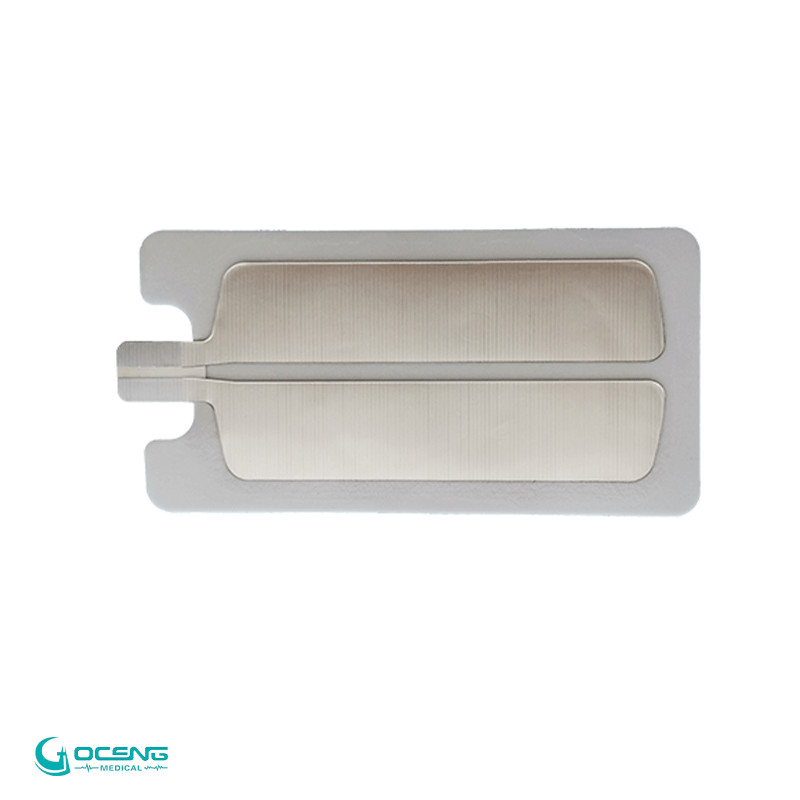
2. Split-Type (REM) Monitoring Safety
Dual-zone structure enables real-time contact monitoring, significantly reducing burn risk and meeting international surgical safety standards.
3. Ultra-Soft Backing Material
Flexible foam and non-woven substrates maintain adhesion during position changes—supine, prone, lateral, or traction positioning.
4. Moisture & Sweat Resistance
Dedicated designs for humid surgical environments, particularly urology and laparoscopic procedures.
5. Twelve Models for All Clinical Scenarios
GP-001 to GP-012 series includes:
Standard adult pads
Extended-size adult pads
Pediatric-specific models
Long-cable versions
High-power reinforced models
Ultra-soft variants for gynecology/pediatrics
Universal international-compatibility models
6. Global Plug-and-Play Compatibility
Fully compatible with mainstream electrosurgical generators across Europe, America, Asia, and Latin America.
Scene-Based Product Applications
✔ GP-001 - GP-002 — General & Cancer Surgery
High-conductivity hydrogel with reinforced foil for extended, high-power procedures.
✔ GP-003 - GP-004 — Gynecology & Sensitive-Skin Surgeries
Soft substrate and hypoallergenic hydrogel for enhanced skin comfort.
✔ GP-005 — Pediatric Model
Smaller size, lower impedance, safe for children’s delicate skin.
✔ GP-006 / GP-007 — Moisture-Resistant Models
Anti-sweat construction ideal for urology and long laparoscopic procedures.
✔ GP-001 / GP-012 — Universal Compatibility Models
Suitable for hospitals standardizing across mixed ESU brands.
✔ GP-010 / GP-011 / GP-012 — Extended & High-Power Versions
Designed for orthopedic, bariatric, and distant-site surgical positioning.
Commitment to Global OR Safety
Through continuous innovation in hydrogel chemistry, material engineering, and REM safety monitoring, Goceng Medical is dedicated to offering reliable and safe solutions for operating rooms worldwide.
The launch of the GP-001 to GP-012 Neutral Electrode Series reinforces the company’s position as a trusted partner in surgical energy management.